Hydrogen is what stars are made of. Today I will show you how to split water into hydrogen and oxygen at home using a simple method and things from your kitchen.
How to split water into hydrogen and oxygen at home
Even being the third most abundant element on our planet’s surface hydrogen is not easy to get. As a gas hydrogen is very rare in our atmosphere and can be found mostly on the upper layers. In chemical compounds hydrogen can be found on our planet surface in hydrocarbons and water.
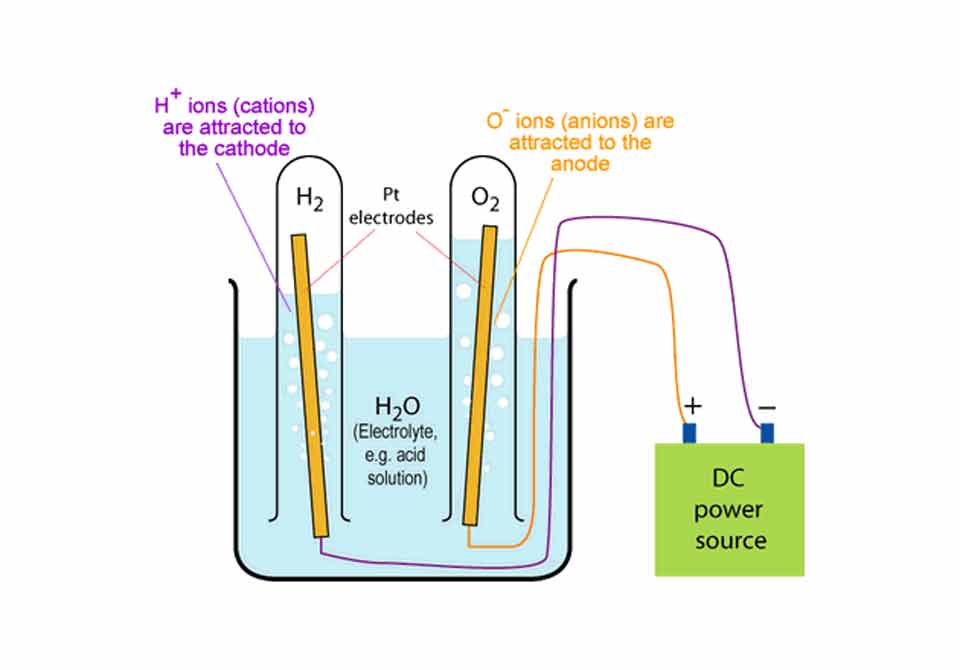
Water can be split into hydrogen and oxygen passing a direct current through a water electrolyte. Today I will explain you how to split water into hydrogen and oxygen at home through electrolysis.
Materials needed:
- Plastic or glass jar (any container which can hold water and you can see through)
- 2 graphite electrodes (also stainless steel or any other metal – 2 nails)
- water (the hydrogen will be extracted from this water)
- 9V or 12V battery (or a low voltage DC power supply)
- copper wires (stainless steel wires are better but harder to bend)
- Optionally baking soda or any other salt which would make the electrolyte more conductive
After you gathered all the materials let’s make some hydrogen. Let’s see how to split water into hydrogen and oxygen at home in your own kitchen.
The electrolyzer
You can find graphite electrodes in any battery. The 6V battery has 3 graphite electrodes inside. Open it carefully, extract the electrodes and clean them properly.
Connect the wires to the graphite electrodes and position the electrodes in the jar in such a way that they stay close to each other nut do not touch. Ideally you should place them parallel spaced with a 0.2 inch (3-5 mm) gap.
The electrolyte
The electrolysis can be done with just tap water but if you want more hydrogen (more bubbles) you need to increase the conductivity of your electrolyte. Just add a full spoon of baking soda to one litter of water and stir till the baking soda dissolves.
Making hydrogen
Let’s actually see how to split water into hydrogen and oxygen at home: Pour the water (the electrolyte) in the jar till it covers your graphite electrodes completely. Connect the wire coming from one electrode to the positive battery terminal and the other wire coming from the other electrode to the negative terminal of the battery (or of the DC power supply).
Once you made the electrical connection you will see bubbles start forming on both electrodes. You actually see water split into hydrogen and oxygen.
Water will split into it’s components. Hydrogen will form on the negative electrode and the oxygen will appear on the positive.
Collecting the gases
First turn off the power by disconnecting the battery or turning off the DC power supply.
In case you need to collect the hydrogen and oxygen for another experiment or just for fun you can fill up a sample tube with electrolyte and place it in the jar upside down making sure you don’t let any air inside. Carefully insert one electrode inside the sample tube and connect the wires to the battery (or just turn on the DC power supply).
Bubbles will start forming around the electrode and then will go up filling up the upside down placed sample tube.
If you need to know how to split water into hydrogen and oxygen at home and collect both gases just place each electrode inside one of the test tubes full of electrolyte, placed upside down inside the jar. Both test tubes will start filling up.
A little warning sign!
It can be fun as long as you gather small amounts of bubbles (a full spoon for example). Adding just a small droplet of liquid soap or shampoo to the electrolyte would help bubbles form on top and when you have a decent amount you can lit them with a lighter causing a small explosion, noisy though.
Why it explodes?
While making the electrolysis is simple and safe, storing the resulted gas is another story. Just continue reading how to split water into hydrogen and oxygen at home and stay safe.
Hydrogen combines with all non metal elements in the periodic table. Oxygen also oxidizes almost all the elements in the periodic table. While you split the water through electrolysis these gases mix with air and stay in very low concentrations which are not dangerous.
Once you store both gases together you are not on the safe side any more. Any spark, any electrostatic discharge could trigger the explosion. If stored in a glass container it could break and shatter. Please always use safety glasses and gloves while you’re experimenting how to split water into hydrogen and oxygen at home.
Increasing efficiency of electrolysis
We already increased a lot the efficiency of tap water electrolysis by adding baking soda salt to it. We created a more conductive electrolyte and so increased the productivity.
Electrolysis enthusiasts believe that pulsed direct current at specific frequencies increases the efficiency of splitting water into it’s components. It all started with Stanley Meyer which claimed he was able to run a car just on water. Stanley Meyer’s secret method went with him to his grave.
Lately scientists were working on how to split water into hydrogen and oxygen using light, silicon electrolyte and semiconductor electrodes. The research is still undergo but it promises to be a breakthrough.
Final thoughts
Explaining how to split water into hydrogen and oxygen at home gave me the idea to make another article called How to make water from hydrogen and oxygen. Please check it out.









[…] in 2008 they discovered how to make a car that runs on water. The invention of a new way to make hydrogen from water. They used a membrane electrode assembly to disrupt the hydrogen from oxygen using a chemical […]